Polar and Nonpolar Molecules
As such it. A notable exception is carbon monoxide CO.

Covalent Bonds Biology For Non Majors I Covalent Bonding Chemical Bond Study Chemistry
Most carbon compounds are nonpolar.
. Amino acids can be divided into two groups based on the polarity as polar amino acids and nonpolar amino acids. The molecule would be polar if it possessed zero dipole moment. There are two types of covalent bonds.
It is possible because of the electrical charges pulling on different parts of the solute molecules. Are ionic bonds polar or nonpolar. Another example is boron trifluoride which is trigonal planar.
Key Difference Polar vs Nonpolar Amino Acids. Polar molecules occur when two atoms do not share electrons equally in a covalent bondA dipole forms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. Examples of Non-polar.
In general pyramid-shaped and V-shaped molecules are said to be polar. Polar solvent is a type of solvent that has large partial charges or dipole moments. Dipoles on one polar molecule can interact with other dipoles on other polar molecules in a process called dipole-dipole interaction.
Lipid-soluble nonpolar molecules pass readily through a cell membrane because they dissolve in the hydrophobic nonpolar portion of the lipid bilayer. Typically the designation of whether a molecule is polar or nonpolar comes from the sum of all of its bonds considered together. Is Water H2O a Polar or Nonpolar Molecule.
Polar molecules are formed either as a result of electronegative atoms or due to asymmetric arrangement of nonpolar bonds and lone pairs of electrons on the same molecule. In the case of polar molecules like attracts like polar molecules tend to interact strongly with other polar molecules because their positive and negative ends are attracted to each other. In polar covalent electron pair is pulled more by one atom compared to the other atom.
The electronegativity values are equal or nearly equal. Molecules containing polar bonds have no. A polar head and a nonpolar tail Due to this the polar heads interact with water while the nonpolar tails try to avoid water and.
The division of a sample of a substance into progressively smaller parts produces no change in either its composition or its chemical properties until parts. Water is said to be a polar molecule due to the difference. Nonpolar molecules usually will dissolve well in nonpolar solvents but tend to be insoluble in water.
Hydrogen atoms in polar bonds. Although permeable to water a polar molecule the nonpolar lipid bilayer of cell membranes is impermeable to many other polar molecules such as charged ions or those that contain many polar. The carboxyl group of one amino acid and the amino group.
The sequence and number of amino acids ultimately determine a proteins shape size and function. Molecule a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition and chemical properties of that substance. Whereas the Linear molecules are said to be non-polar in nature.
The bonds between the atoms have very different but measurable electronegativities. Covalent molecules made of only one type of atom like hydrogen gas H2 are nonpolar because the hydrogen atoms share their electrons equally. They possess symmetrical polar bonds.
The difference between polar and nonpolar molecules can thus be found by the vectors of partial charge resulting from each bond. A polar solvent can dissolve ions and other polar compounds. By comparing their EN difference we can say carbon-phosphorus is nonpolar bond.
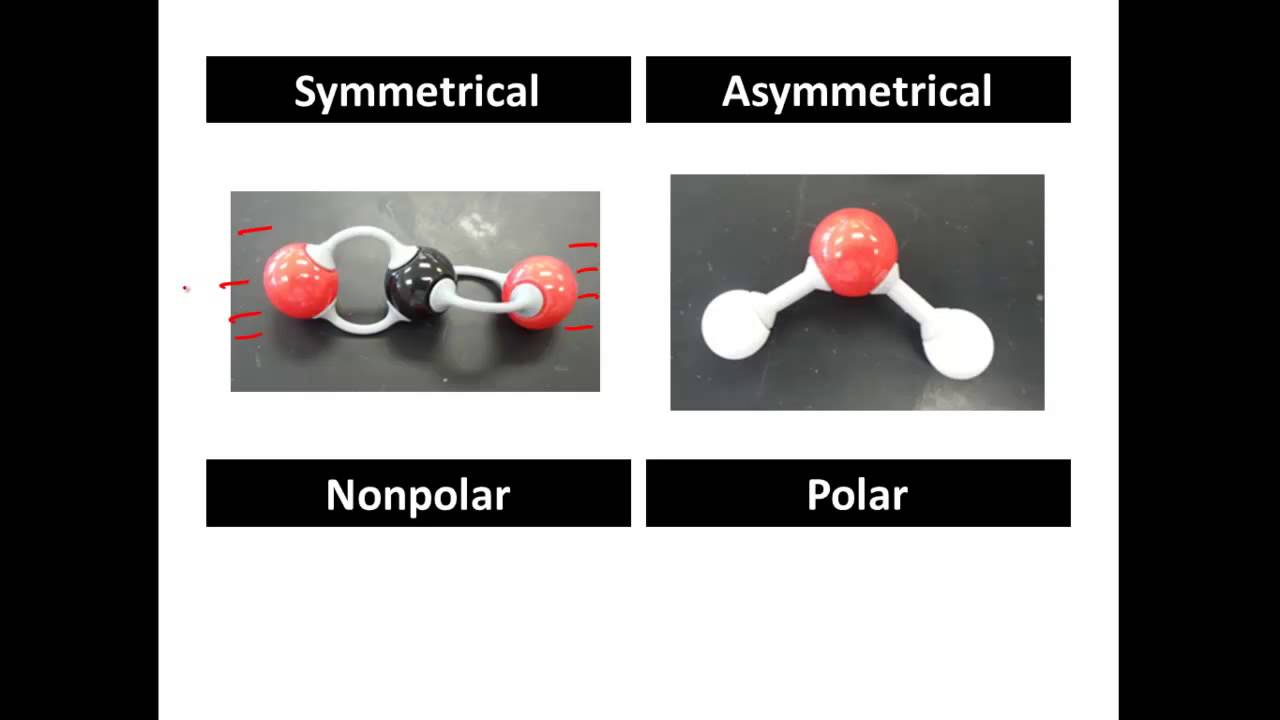
Differences Between Polar Nonpolar in Chemistry. In symmetrical molecules the dipole charges cancel out. The following examples will explain the two phenomena in a more elaborated manner.
This means that polar molecules can more easily chemically bond with other polar molecules. Theres no element with more negative or positive charge than another side. Is SeCl 2 a polar or nonpolar molecule.
Hydrogen bonds can form between different molecules and they do not always have to include a water molecule. Amino acid are organic compounds. Examples of homonuclear nonpolar molecules are oxygen O 2 nitrogen N 2 and ozone O 3.
A non-polar molecule has a structure of its atoms lined up in a way that the orbital electrons in the outer region cancel out the electronegativity. Figure 27 Hydrogen bonds form between slightly positive δ and slightly negative δ charges of polar covalent molecules such as water. Symmetrical molecules are nonpolar.
The key difference between polar and nonpolar amino acids is that polar amino acids have polarity whereas polarity is absent in nonpolar amino acids. Each amino acid is attached to another amino acid by a covalent bond known as a peptide bond which is formed by a dehydration reaction. This video provides a fast way for you to determine if a molecule is polar or nonpolar.
41 Biological Molecules. This happens when there is a difference between the electronegativity values of each atom. Because nonpolar molecules share their charges evenly they do not react to electrostatic charges like water does.
Polar covalent bonds are made by two atoms with different electronegativities but the different should not be exceeding 17. Polar compounds are asymmetrically arrayed. Some chemical species such as chains of carbon molecules share electrons equally and are said to be nonpolar molecules.
Nonpolar molecules are generally symmetrical like the tetrahedral molecule carbon tetrachloride. Many gases like hydrogen helium oxygen carbon dioxide and nitrogen are some of the particular examples of nonpolar molecules. If they are highly different it can be said that the species is a highly polar molecule.
The electronegativity difference between bonded atoms is less than 04. The partial positive and partial negative poles are present across the molecule as a result. Water is a polar molecule since it is formed using a strongly electronegative Oxygen atom that pulls a pair of the Hydrogen atoms and possesses a slightly negative charge.
It provides examples so you can quickly distinguish nonpolar molecul. How to Explain Polarity. A membrane protein likely has to interact with both the nonpolar interior and the polar exterior of the phospholipid membrane.
Non-polar molecules have a significant dipole moment value. Molecules come in infinite varieties so in order to help the complicated chemical world make a little more sense we classify and categorize them. How to Determine Polarity in Chemistry.
In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment with a negatively charged end and a positively charged end. Other nonpolar molecules include carbon dioxide CO 2 and the organic molecules methane CH 4 toluene and gasoline. Nonpolar molecules can bond with nonpolar molecules but it is not quite as easy for them.
In non-polar covalent bonds electrons are equally shared by the two atoms participating in making the bond. Polar Molecules. An extreme difference forms an.
In the act of surrounding the polar molecules of another substance water wriggles its way into all the nooks and crannies between molecules effectively breaking it apart are dissolving it. Nonpolar and polar molecules exhibit some degree of electronegativity difference between bonded atoms1 Nonpolar bonds. 3-6 for examples of polar and.
SeCl 2 is a polar molecule because it has an EN difference greater than 04 among atoms. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. This happens because the.
Polar molecules possess equal distribution of electron density.

Polar And Non Polar Covalent Molecules Polar Vs Nonpolar Youtube Playlist Science Chemistry Science Activities Chemistry

Covalent Bonds Biology For Non Majors I Covalent Bonding Chemical Bond Study Chemistry

Polar Nonpolar Covalent Bonds Ch 6 Youtubea Little Too Detailed Though Covalent Bonding Chemistry Classroom Chemistry Lessons

Polar And Nonpolar Molecules Covalent Bonding Chemistry Lessons Chemistry Classroom


0 Response to "Polar and Nonpolar Molecules"
Post a Comment